Graduate School of Agriculture
Kindai University
Kindai University
Staff

Tsutomu Kawasaki, Ph
Professor
< Contact Information >
Laboratory of Plant Molecular Genetics
Department of Advanced Bioscience
Graduate School of Agriculture Kindai University
3327-204 Nakamachi, Nara 631-8505, Japan
TEL/FAX 81-743-42-7335
e-mail: t-kawasaki [at] nara.kindai.ac.jp
Koji Yamaguchi, Ph D
Assistant professor
TEL 81-743-42-1601
FAX 81-743-42-7335
e-mail: k-yamaguchi [at] nara.kindai.ac.jp
Research
Plant Immune System
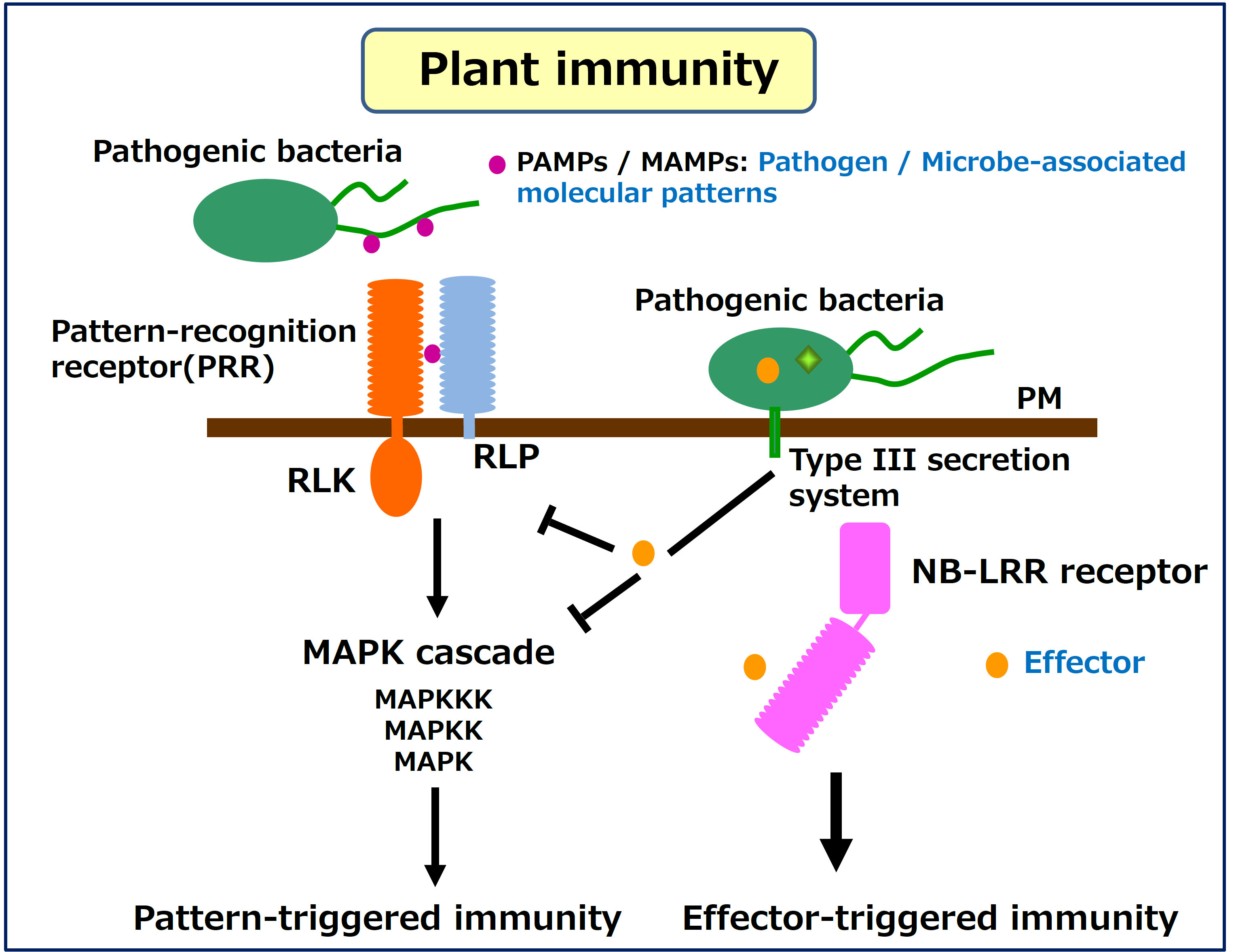 The plant innate immunity system is generally initiated via recognition
of conserved pathogen / microbe-associated molecular pattern (PAMP / MAMP)
ligands, including bacterial flagellin or peptidoglycan, and fungal chitin.
These MAMPs are perceived by pattern recognition receptors (PRRs), leading
to activation of a series of immune responses (PTI: pattern-triggered immunity).
PRRs are classified into two types of receptors, receptor-like kinase (RLKs)
and receptor-like proteins (RLP). Both types of PRRs contain ligand-binding
domains and a transmembrane domain, but only RLKs have an intercellular
kinase domain. The well-characterized ligand-binding domains are LRR (leucine-rich
repeat) and LysM (lysine rich motif).
The plant innate immunity system is generally initiated via recognition
of conserved pathogen / microbe-associated molecular pattern (PAMP / MAMP)
ligands, including bacterial flagellin or peptidoglycan, and fungal chitin.
These MAMPs are perceived by pattern recognition receptors (PRRs), leading
to activation of a series of immune responses (PTI: pattern-triggered immunity).
PRRs are classified into two types of receptors, receptor-like kinase (RLKs)
and receptor-like proteins (RLP). Both types of PRRs contain ligand-binding
domains and a transmembrane domain, but only RLKs have an intercellular
kinase domain. The well-characterized ligand-binding domains are LRR (leucine-rich
repeat) and LysM (lysine rich motif). Successful pathogens are able to secrete or directly deliver effector proteins into the host cytoplasm which results in inhibition of host PTI. To overcome PTI inhibition by the effectors, plants have developed intracellular immune receptors of the nucleotide-binding leucine-rich repeat (NB-LRR) protein family, which directly or indirectly recognize the effectors, and activate strong immune responses (ETI: effector-triggered immunity).
Perception of PAMPs with PRRs induces a rapid intracellular activation of mitogen-activated protein kinase (MAPK) cascades consisting of highly conserved modules in eukaryotes. Each MAPK cascade is composed of three consecutively acting protein kinases: a MAPK kinase kinase (MAPKKK), a MAPK kinase (MAPKK) and a MAPK. The MAPK cascades induce robust immune responses, however how the MAPK cascades are activated downstream of PRRs is largely unknown. We are interested in understanding how PRRs and NB-LRR receptors transmit the immune signals to the downstream components to induce PTI and ETI, respectively, and how the pathogen effectors inhibit host immune response.
Current Projects
1)Elucidation of molecular mechanism of pattern-triggered immunity in plants.PRRs recognize PAMPs at plasma membrane, which triggers the intracellular immune response. However, the molecular mechanisms remain to be identified. Our research focuses on immune signaling induced by recognition of fungal chitin and bacterial peptidoglycan. Rice CEBiP and LYP4/LYP6 are RLPs with the extracellular LysM domains responsible for recognition of chitin and PGN, respectively. Perception of chitin with CEBiP induces a complex formation with a RLK OsCERK1, which activates the intracellular immune responses.
We have identified that a receptor-like cytoplasmic kinase (RLCK) OsRLCK185 interacts with the cytoplasmic kinase domain of OsCERK1. In addition, chitin perception triggers OsCERK1-mediated phosphorylation of OsRLCK185. Silencing of OsRLCK185 suppresses immune responses induced by recognition of chitin and PGN, indicating that OsRLCK185 plays a key role in OsCERK1-mediated immune signaling (Yamaguchi et al. Cell Host & Microbe 2013: the paper was selected as the featured article).
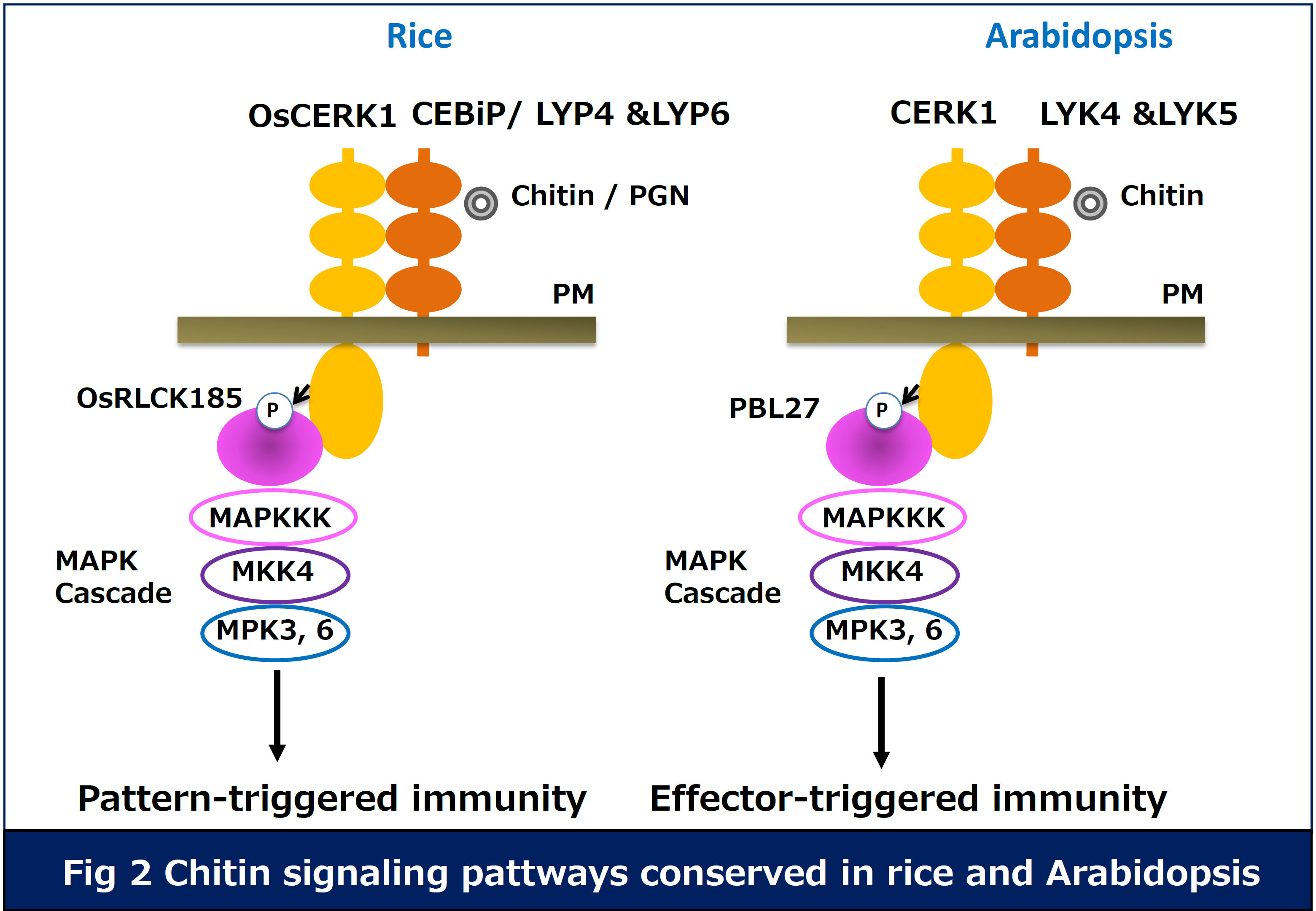 We also identified PBL27, Arabidopsis homolog of OsRLCK185. PBL27 interacts
with CERK1 at plasma membrane, and is phosphorylated by CERK1 in response
to chitin. Our data indicate the chitin-signaling pathways are conserved
in rice and Arabidopsis (Fig. 2). These results were obtained with collaboration
of Dr. Shibuya group (Meiji Univ) (Shinya and Yamaguchi et al. Plant J.
2014). Currently, we are analyzing how CERK1 activates PBL27 and how PBL27
induces intracellular immune responses.
We also identified PBL27, Arabidopsis homolog of OsRLCK185. PBL27 interacts
with CERK1 at plasma membrane, and is phosphorylated by CERK1 in response
to chitin. Our data indicate the chitin-signaling pathways are conserved
in rice and Arabidopsis (Fig. 2). These results were obtained with collaboration
of Dr. Shibuya group (Meiji Univ) (Shinya and Yamaguchi et al. Plant J.
2014). Currently, we are analyzing how CERK1 activates PBL27 and how PBL27
induces intracellular immune responses. 2)Understanding the inhibitory mechanisms by pathogen effectors
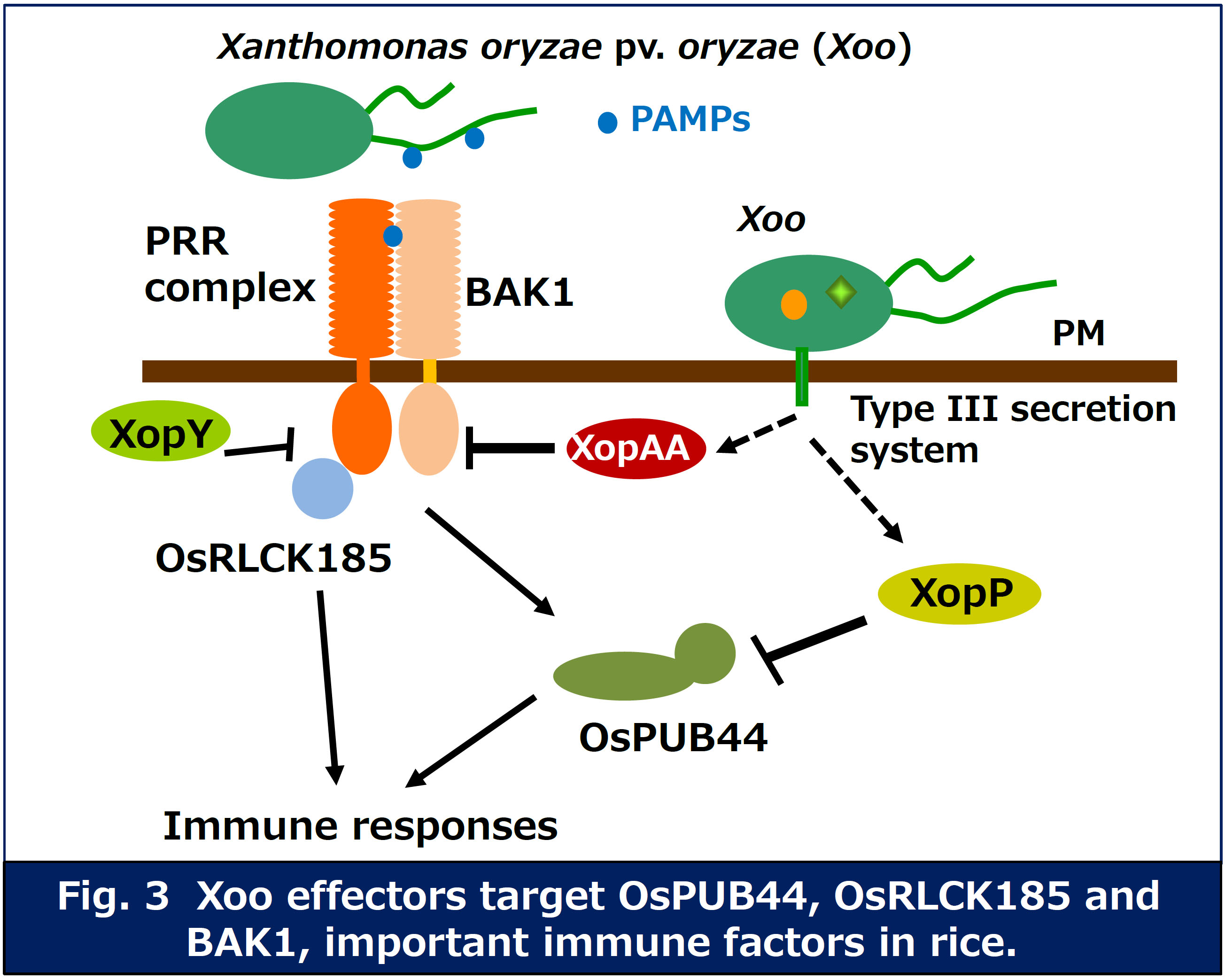 Pathogen effectors are secreted into host cells and inhibit the immune
responses. In fact, expression of Xanthomonas oryzae pv. oryzae (Xoo) effectors
in rice cells results in inhibition of PTI, suggesting that the effectors
target important immune factors. We have identified several immune factors
interacted with the effectors by yeast two hybrid screening. We are currently
analyzing the functions of these factors in plant immune response, and
inhibitory mechanism of host immunity by the pathogen effectors (Fig. 3).
Pathogen effectors are secreted into host cells and inhibit the immune
responses. In fact, expression of Xanthomonas oryzae pv. oryzae (Xoo) effectors
in rice cells results in inhibition of PTI, suggesting that the effectors
target important immune factors. We have identified several immune factors
interacted with the effectors by yeast two hybrid screening. We are currently
analyzing the functions of these factors in plant immune response, and
inhibitory mechanism of host immunity by the pathogen effectors (Fig. 3).
We have identified a rice receptor-like cytoplasmic kinase (OsRLCK185) targeted by Xoo effector XopY (Xoo1488). Our research indicates that XopY suppresses chitin-induced immunity by inhibiting the phosphorylation of OsRLCK185 by OsCERK1 (Yamaguchi et al. Cell Host & Microbe 2013).
We also found that a Xoo effector, XopP (Xoo3222) targets OsPUB44, one of rice U-box type ubiquitin E3 ligases. OsPUB44 functions as a positive regulator in chitin- and PGN-induced immunity. The U-box domain of OsPUB44 possesses the ubiquitin ligase activity. XopP interacts with the U-box domain, which results in suppression of the OsPUB44 enzymatic activity. These data indicate that XopP modulates host ubiquitination system to inhibit the immunity (Ishikawa et al. Nature Communications 2014).
3)Elucidation of molecular mechanism of effector-triggered immunity in plants
The intracellular NB-LRR receptors recognize pathogen effectors in the cells, and induce immune responses often accompanied with hyper-sensitive cell death. Rice Xa1 is one of rice NB-LRR receptors (Yoshimura et al. Proc. Natl. Acad. Sci. USA 1998), which possibly recognizes directly or indirectly Xoo AvrXa1. However, AvrXa1 has not been identified yet. We are analyzing Xa1-mediated pathogen recognition and immune signaling by isolation of rice factors interacted with Xa1.
4)Understanding of MAPK activation in plant immunity
The MAPK cascades are activated by PAMP perception with PRRs. However, it is unknown how PRRs transmit immune signals to MAPK cascades. As mentioned above, OsRLCK185 and PBL27 regulate chitin-induced MAPK activation. Recently, we isolated MAPKKKs interacted with OsRLCK185 and PBL27. We are studying the molecular mechanisms of RLCK-mediated MAPK activation in plants.
5) Screening of chemicals that activate host immunity or inhibit the virulence of pathogens
Based upon our data, we developed the screening system to identify new pesticides that induce host immunity or inhibit the proliferation of the pathogens. The use of these chemicals likely contributes to the environmental conservation.
6) New technology to improve plant disease resistance
During the past several decades, plant Immune research has identified many important genes regulating host immunity from model plants such as Arabidopsis and rice. Recently, the genes were used to develop broad-spectrum and durable resistance to pathogens. This type of research is termed as ‘translational research’.
☆☆☆
☆☆
近畿大学農学部
バイオサイエンス学科
植物分子遺伝学教室
ビルダークリニック
〒631-8505
奈良県奈良市中町3327-204
TEL 0742-43-7335
FAX 0742-43-7335